Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Screening and Production of Extracellular Fungal Laccase from Peniophora Albobdia by Solid State Fermentation with Agro-industrial Waste
Authors: Komal Solanke, Aparna Taware
DOI Link: https://doi.org/10.22214/ijraset.2025.66953
Certificate: View Certificate
Abstract
The enzyme Laccase is known to degrade many phenolic, aromatic compounds. The present study describes, fungal fruiting body collected from Chhatrapati Sambhajinagar, isolated on 2% Malt Extract Agar and substrate oxidation studies carried out to screen fungi for laccase production by using different laccase substrate. The aim of this study was to extract extracellular laccase from Peniophora albobdia with solid state fermentation by different agro industrial wastes like Tur bagasse, Corn stubs, Soybean bagasse, Groundnut bagasse, sugarcane bagasse, and brewer’s spent grain. A utilization of biomass waste had applied in this research. The enzyme activity unit of the different types of substrates was significantly different. The highest activity unit was obtained by Brewers spent grain than the other substrates. From results it is confirmed that the best substrate for laccase production is brewers spent grain with nutrient addition. The statistical optimization needs to be carried to increase the production of laccase enzyme which will help to bio-eliminate the various industrial phenolic waste compounds.
Introduction
I. INTRODUCTION
Laccase (benzenediol: oxygen oxidoreductase; EC 1.10.3.2) is a blue-copper oxidoreductase that catalyse the one electron oxidation of wide range of substrates including phenolic compounds with the concomitant reduction of molecular oxygen to water. Most biotechnologically useful laccases are of fungal origin especially from the classes of white-rot fungi however, the enzyme has been found in plants, insects and some bacteria but Due to the higher redox potential of fungal laccase compared to plant or bacterial laccase, they are utilized in several biotechnological applications (Nunes and Kunamneni, 2018). Laccase considered as “green tool” because it is able to perform catalysis using molecular oxygen as co-substrate rather than the hydrogen peroxide like other oxidoreductase and it yield water as a by-product. Laccases are currently highly interesting enzyme because of their broad substrate specificity. The problem associated to industrial fungal laccase exploitation is the low production by native host. The effective use of industrial laccases may be hindered by their non-reusability, and high sensitivity to denaturing agents. Many attempts have been made to improve the storage and operational stability of laccase, leading to lower cost and expansion of its biotechnological and environmental applications (Geethanjali & Jayashankar, 2018).
Enzyme production is highly regulated by nutrients, Carbon and nitrogen sources as well as microelements. The production of the enzyme can be influenced by the type of culture (submerged or solid state). The ligninolytic machinery in white rot fungi have strong regulating effects metabolism (Brijwani et al., 2010). This information provides data for several approaches which were used to promote enzyme production. Lignocellulose-derived wastes having abundant carbon sources produced in many agro-industrial processes and is one of the most attractive bio-based materials with potential for being a source of enzymes. These wastes are attractive due to their renewable nature, abundance and low cost (De Castro & Sato, 2015; Soccol et al., 2017). In most studies laccase have been isolated from fungi capable of destroying wood, especially white-rot fungi such as: Pleurotus pulmonarius, Pleurotus ostreatus, Agaricus bisporus, Trametes versicolor, etc. Fungal laccases play an important role in plant pathogenesis, pigment production, and degradation of lignocellulosic materials (Bertrand et al., 2016; Nunes and Kunamneni, 2018). Biotechnological applications require large amounts of enzymes at low cost. However, current commercial enzymes are still expensive due to the low yield and high production and isolation costs. Therefore, the tasks should point toward searching for new hyper secretory strains and enhancing cultural conditions (L. Villalba et al., 2010). The aim of this research work is to isolate and screen the laccase producing fungi using different laccase enzyme indicator compounds and identifying new potent extracellular laccase source.
II. METHODS AND MATERIAL
A. Chemicals
Malt extract agar, Agar powder, ABTS 4, 4’- [azino bis (methanylylidene)] bis (2, 6-dimethoxyphenol), Guaiacol, tannic acid, α-naphthol, purchased from Sigma Aldrich and Hi media Mumbai. All chemicals used in this research work were certified reagent grade.
B. Collection and isolation of fungal strain
Fruiting bodies of fungal samples collected in sterile plastic bag from walking plaza, Ayodhya Nagari, Padampura, Chhatrapati Sambhajinagar. Fungi were isolated from fruiting bodies by tissue culture technique. The fungal sample was sterilized using 0.1% mercuric chloride for 30 s under aseptic condition. Further the fungus was washed with sterilized distilled water for 3-4 times, then sterilized with 75% alcohol under aseptic conditions for 30 s and then washed with distilled water after that inoculated on 2% MEA (Malt Extract Agar) medium. Streptomycin (200 mg/L) was added as an antibacterial agent. Complete MEA medium was sterilized in an autoclave for 20 min at 121 ºC (Aisha Umar 2021). Marginal sections of fruiting bodies were used as inoculating material. Sub cultured as pure mycelial culture on MEA medium (Mishra et al., 2011).
C. Screening of fungi for laccase production
The pure culture tested for laccase production on different laccase activity indicator substrate by plate test assay method. 2% malt agar extract supplemented with guaiacol (0.02%) (wt/wt), 1-naphthol (5 mm), tannic acid (0.5%) (wt/wt) and ABTS 0.1 % (wt/wt) then inoculated with fungal culture were used for screening of laccase production by the selected fungal species. These Petri plates were incubated at 28–30 °C for 72 h and then screened for the formation of coloured zones around the fungal colonies (Mishra et al., 2011).
D. Production of laccase enzyme
1) Submerged Fermentation
Fungal culture which exhibited color change were used for further growth. Standard inoculum (disk of fungal growth 2 mm) of the most potent fungus was cultivated into 250 ml flasks containing 100 ml of productive liquid medium. which contained the following: 3 g peptone, 10 g glucose, 0.6 g KH2PO4, 0.001 g ZnSO4, 0.4 g K2HPO4, 0.0005 g FeSO4, 0.05 g MnSO4 and 0.5 g MgSO4 per L, pH 5.5, and then incubated at 30 °C for 12 days on rotary shaker (150 rpm). Fungal growth and enzyme activity were assayed.
2) Solid state Fermentation
The substrates that used in this method were sugar bagasse, Tur bagasse, Corn stubs, Soybean bagasse, Groundnut bagasse and brewer’s spent grain. The medium was supplemented with nutrient. The components of nutrients were (g/L sulphate acid pH 5) NH4NO3—3; (NH4)2HPO4—3; KH2PO4—5; MgSO4.7H2O—0.5; NaCl—0.5; CaCO3— 0.5; FeSO4.7H2O—0.35; CuSO4.5H2O—0.6(Perdani et al., 2020).
E. Enzyme Extraction And Laccase Activity Measurement
Enzymes were extracted from the fermented substrate. The fermented product is mixed with 50 mL of sodium acetate buffer (50 mm, pH 5.0). The results of the mixture were stirred with a rotary shaker at a speed of 120 rpm for 60 min. Then, the filtered using muslin cloth and centrifuged at a speed of 9000 rpm for 10 min at 4 ?C. The supernatant obtained is a crude Laccase extract, which is partially tested for the activity of the Laccase enzyme. Supernatant activity test was carried out using a spectrophotometer to determine the enzyme activity (Perdani et al., 2020). Guaiacol used for activity measurement. The reaction mixture for quantification of laccase under spectrophotometer comprised 1 mL crude enzyme supernatant, 3 mL sodium acetate buffer and 1 mL Guaiacol it was incubated for 10 min at 30 °C. After 30 s absorbance was measured at 470 nm (ε465 = 12,100 M−1 cm−1).
E.A = A × V / t × e × v
{E.A = Enzyme activity A = Absorbance V = Total mixture volume (ml) v = enzyme volume (ml) t = incubation time e = extinction coefficient for Guaiacol (0.6740 μM/ cm)}.
III. RESULT
A. Collection and Isolation of Fungal Sample

Figure 1 peniophora albobobdia Figure 2 culture on MEA Plate
The fungal sample collected from dead logs subjected to the morphological identification. Based on the morphological and microscopic characters, it is inferred that the isolated fungus belongs to the class of basidiomycetes white rot fungus Peniophora Cooke (Peniophoraceae, Russulales) is a large corticioid genus characterized by annual Basidiomes, adnate resupinate with a smooth hymenophore, a dimitic hyphal system with both encrusted cystidia and gloeocystidia, and thin-walled smooth basidiospores. Species of the genus prefer to grow on small branches especially those dead but still attached.
B. Screening for Enzyme Production
White rot fungus Peniophora sp. was screened for laccase production on MEA media emended with laccase substrate such as tannic acid, ABTS, Guaiacol and α-naphthol. The oxidation of laccase substrate observed. Extracellular Laccase oxidizes α-naphthol to a deep purple complex, guaiacol to Reddish brown colour, ABTS to deep purple colour and tannic acid to Brown colour giving a visual confirmation for the presence of the Extracellular laccases enzyme as shown in Fig 3.
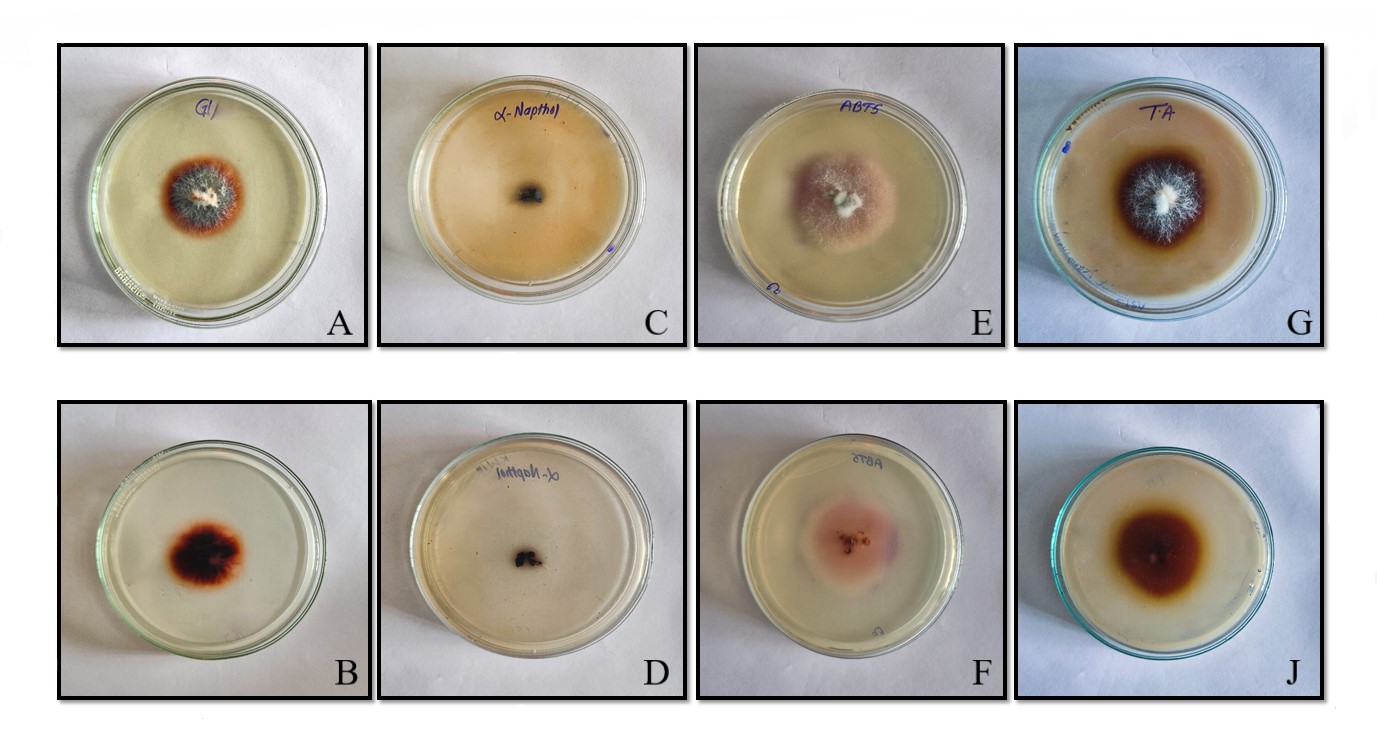 Figure 3. Screening for laccase synthesis by P. albobdia on medium with different substrate. Front and back morphology of α-Naphthol (A B), Tannic acid (C D), Guaiacol (E F), ABTS (G H).
Figure 3. Screening for laccase synthesis by P. albobdia on medium with different substrate. Front and back morphology of α-Naphthol (A B), Tannic acid (C D), Guaiacol (E F), ABTS (G H).
C. Laccase Activity Measurement
After screening for laccase production Peniophora culture was used for laccase production. Peniophora Culture cultivated on different media for quantitative analysis of laccase activity. Laccase activity was determined using guaiacol as substrate. This phenolic compound was oxidized to a more stable cation radical. The concentration of the cation radical responsible for intense reddish brown colour. Initially enzyme production was assayed in submerged medium and solid-state fermentation medium. It was observed that in SSF medium enzyme production was much higher than (0.015 µg/ml) submerged medium (Table No.1.1)
|
Sr.No. |
Media |
Enzyme production µg/ml |
|
1 |
Submerged fermentation |
0.008987±4.860 |
|
2 |
Solid state fermentation |
0.015±0.0021 |
Table 1.1 Production of laccase enzymes by using different fermentation types
SSF was used further for production of enzyme. Different substrates were used in SSF like Tur bagasse, Corn stubs, Soybean bagasse, Groundnut bagasse, sugarcane bagasse, brewer’s spent grain. Enzyme production was higher when brewer’s spent grain was used as substrates. Enzyme production was 0.4044 µg/ml which is near by 20 times higher than other substrates (Table No.1.2).
|
Sr.No. |
Substrate |
Enzyme production µg/ml |
|
Tur bagasse, |
0.0180±0.0008 |
|
|
2 |
Corn stubs |
0.0135±0.0003 |
|
3 |
Soybean bagasse |
0.0112±0.004 |
|
4 |
Groundnut bagasse |
0.0157±0.0008 |
|
5 |
sugarcane bagasse |
0.0090±0.00086 |
|
6 |
brewer’s spent grain |
0.4044±0.00098 |
Table 1.2 laccase production by solid state fermentation using different substrates
This experiment showed significant result, that solid state fermentation is more effective than submerged fermentation and economically affordable also, agro-industrial wastes are more effective for not only laccase production but also effective for other economically important enzyme production.
IV. DISCUSSION
White rot fungus peniophora albobdia isolated from decaying wood. Peniophora is a large corticoid genus shows cosmopolitan distribution, Peniophora is a good example of ecological adaptation to dry and exposed environments, characterized by resupinate basidiomata, presence of meruloids (here called lamprocystidia, or encrusted cystidia) and causes white rot to plants (Rajchenberg et al., 2024; Xu et al., 2023). Peniophora sp screened for laccase production on different laccase indicator compound shows positive results for enzyme production so strain used for further laccase production experiment. Almost all species of white-rot fungi were reported to produce laccase to varying degrees. The fungus must be selected according its enzymatic activities (Farinas, 2015). Dodge (2009) state that commercial lignocellulolytic enzymes are mainly produced through submerged fermentation (SmF) but it demand energy for sterilization, enzymatic solutions obtained from SmF increases the total downstream processing costs. solid state fermentation attractive process for fungal cultivation because it has similar characteristics to natural habitat, low risk of contamination, high yield, utilization of low-cost substrates, simplicity of processing, lower energy requirements and decreased waste-water production make this process more attractive when compared to submerged fermentation (Chen et al., 2014; De Castro & Sato, 2015). It was observed that in SSF medium enzyme production was much higher than (0.015 µg/ml) submerged medium (Table No.1.1). These positive effects on laccase production were similar to those obtained by Liu et al. (2020). . The most important factors to be considered during the development of a SSF are the choice of microorganisms and the choice of substrates. Laccase production by white rot fungi depends on cultivation condition. It directly proportional to growth and more growth means higher laccase production and enzyme activity. Due to this reason growth is the first criteria took in many studies (Abhijit M, 2015). White rot fungi suitable for SSF, since the technique simulates their natural habitat.
In India, large amount waste are produced every year from agricultural activities, these waste disposed of by burning but these waste act as a potential substrate for production of extracellular enzymes like wheat bran (Sharma et al., 2016), Rice husk (Perdani et al., 2020), corn stalk (Astolfi et al., 2019; Lu et al., 2022), Sugarcane (Namnuch et al., 2021), brewery spent grain and sugarcane bagasse (Moran-Aguilar et al., 2021). In this study, fungal species cultivated on solid lignocellulose substrates like Tur bagasse, Corn stubs, Soybean bagasse, Groundnut bagasse, sugarcane bagasse, and brewer’s spent grain to compare their extracellular enzyme production. However not all lignocellulosic waste is suitable for cultivation of fungi, considering the white rot fungus P. albobdia its notable that the production of laccase on BSG higher than the other substrate. highest laccase activity of nearly 0.4044 µg/ml which was about 20 fold higher than that Tur bagasse presented laccase activity of about 0.0180 µg/ml, whereas the values of corn stubs, Soybean bagasse, Groundnut bagasse, Sugarcane bagasse cultures 0.0135 µg/ml, 0.0112 µg/ml, 0.0157 µg/ml, 0.0090 µg/ml respectively were less effective than other substrate. BSG good source for producing enzymes. These positive effects on laccase production were similar to those obtained from other laccase-producing fungi such as Trametes versicolor on Wheat straw and Brewer’s spent grain (Dhillon et al., 2012; Singh et al., 2019) and by Pleurotus ostreatus on Wheat straw (Albornoz et al., 2018). In agreement with our results, Trametes versicolor also showed the highest laccase activity using on BSG, Pleurotus ostreatus on Sugarcane bagasse and Wheat bran, Pyrenophora phaeocomes on Rice straw culture laccase activity 13,506 U/g, 167 U/g, 10,859 U/g 32,450 U/g, respectively (Dhillon et al., 2012; El-Batal et al., 2015; Karp et al., 2012; Rastogi et al., 2016). These results clearly indicated that the strategy of the upscaling process was planned correctly Hence, BSG was selected as a solid substrate for further studies.
This result clearly indicates that the production of laccase by Peniophora albobdia is much higher than the reported range of laccase enzyme produced by WRF. The brewery industry generates large quantities of brewer’s spent grain (BSG), a solid leftover of fermentation process which is primarily used as animal feed. It is rich in growth factors and vitamins. Spent grain low-cost product available throughout year, constituting one of the most studied materials in the bio refinery sector for producing value-added products (Zeko-Piva? et al., 2022). The reuse of spent grain brings economic benefits and reducing pollution. BSG was evaluated as a substrate for the production of polyphenols and the lignin-degrading enzyme laccase using fungal solid-state fermentation by Trametes versicolor. (Tišma et.al. 2018) Present experiment also proved that BSG is best substrate for laccase production. In this study the use of P. albobdia as a high enzyme producer and BSG recommended as a balanced substrate for microbial growth and cheap growth media to reduce the cost of laccase production.
Conclusion
The present study highlights the laccase producing fungal resource. Study clearly indicates that Peniophora albobdia isolated from fruiting bodies grows on logs of tree is promising source of laccase production. The need for more easily available, cheap substrate meets by Brewer’s Saint Grain (BSG) by producing more laccase than the other substrates. In future we are interested to scale up optimization of various cultural conditions and nutritional parameters for high production of laccase on agro-industrial wastes by solid state fermentation. Media optimization and use of appropriate inducers could bring additional benefits of higher production with expenditure of minimum resources. This stain seems to be a prospective organism for further biotechnical application.
References
[1] Abhijit M, A. C. (2015). Screening and Isolation of Laccase Producers, Determination of Optimal Condition for Growth, Laccase Production and Choose the Best Strain. Journal of Bioremediation & Biodegradation, 06(04). https://doi.org/10.4172/2155-6199.1000298 [2] Albornoz, S., Wyman, V., Palma, C., & Carvajal, A. (2018). Understanding of the contribution of the fungal treatment conditions in a wheat straw biorefinery that produces enzymes and biogas. Biochemical Engineering Journal, 140, 140–147. https://doi.org/10.1016/j.bej.2018.09.011 [3] Astolfi, V., Astolfi, A. L., Mazutti, M. A., Rigo, E., Di Luccio, M., Camargo, A. F., Dalastra, C., Kubeneck, S., Fongaro, G., & Treichel, H. (2019). Cellulolytic enzyme production from agricultural residues for biofuel purpose on circular economy approach. Bioprocess and Biosystems Engineering, 42(5), 677–685. https://doi.org/10.1007/s00449-019-02072-2 [4] Bertrand, B., Mayolo-Deloisa, K., González-González, M., Tinoco-Valencia, R., Serrano-Carreón, L., Martínez-Morales, F., Trejo-Hernández, M. R., & Rito-Palomares, M. (2016). Pleurotus ostreatus laccase recovery from residual compost using aqueous two-phase systems: Recovery of laccase from P. ostreatus using ATPS. Journal of Chemical Technology & Biotechnology, 91(8), 2235–2242. https://doi.org/10.1002/jctb.4995 [5] Brijwani, K., Rigdon, A., & Vadlani, P. V. (2010). Fungal Laccases: Production, Function, and Applications in Food Processing. Enzyme Research, 2010, 1–10. https://doi.org/10.4061/2010/149748 [6] Chen, H.-Z., Liu, Z.-H., & Dai, S.-H. (2014). A novel solid state fermentation coupled with gas stripping enhancing the sweet sorghum stalk conversion performance for bioethanol. Biotechnology for Biofuels, 7(1), 53. https://doi.org/10.1186/1754-6834-7-53 [7] De Castro, R. J. S., & Sato, H. H. (2015a). Enzyme Production by Solid State Fermentation: General Aspects and an Analysis of the Physicochemical Characteristics of Substrates for Agro-industrial Wastes Valorization. Waste and Biomass Valorization, 6(6), 1085–1093. https://doi.org/10.1007/s12649-015-9396-x [8] Dhillon, G. S., Kaur, S., & Brar, S. K. (2012). In-vitro decolorization of recalcitrant dyes through an ecofriendly approach using laccase from Trametes versicolor grown on brewer’s spent grain. International Biodeterioration & Biodegradation, 72, 67–75. https://doi.org/10.1016/j.ibiod.2012.05.012 [9] Dodge, T. (2009). Production of Industrial Enzymes. In R. J. Whitehurst & M. Van Oort (Eds.), Enzymes in Food Technology (1st ed., pp. 44–58). Wiley. https://doi.org/10.1002/9781444309935.ch3 [10] El-Batal, A. I., ElKenawy, N. M., Yassin, A. S., & Amin, M. A. (2015). Laccase production by Pleurotus ostreatus and its application in synthesis of gold nanoparticles. Biotechnology Reports, 5, 31–39. https://doi.org/10.1016/j.btre.2014.11.001 [11] Farinas, C. S. (2015). Developments in solid-state fermentation for the production of biomass-degrading enzymes for the bioenergy sector. Renewable and Sustainable Energy Reviews, 52, 179–188. https://doi.org/10.1016/j.rser.2015.07.092 [12] Geethanjali, P. A., & Jayashankar, M. (n.d.). Efficiency of Paddy Residues as Substrates for Fungal Laccase Production. [13] Karp, S. G., Faraco, V., Amore, A., Birolo, L., Giangrande, C., Soccol, V. T., Pandey, A., & Soccol, C. R. (2012). Characterization of laccase isoforms produced by Pleurotus ostreatus in solid state fermentation of sugarcane bagasse. Bioresource Technology, 114, 735–739. https://doi.org/10.1016/j.biortech.2012.03.058 [14] L. Villalba, L., I. Fonseca, M., Giorgio, M., & D. Zapata, P. (2010). White Rot Fungi Laccases for Biotechnological Applications. Recent Patents on DNA & Gene Sequences, 4(2), 106–112. https://doi.org/10.2174/187221510793205728 [15] Liu, J., Yang, J., Wang, R., Liu, L., Zhang, Y., Bao, H., Jang, J. M., Wang, E., & Yuan, H. (2020). Comparative characterization of extracellular enzymes secreted by Phanerochaete chrysosporium during solid-state and submerged fermentation. International Journal of Biological Macromolecules, 152, 288–294. https://doi.org/10.1016/j.ijbiomac.2020.02.256 [16] Lu, X., Li, F., Zhou, X., Hu, J., & Liu, P. (2022). Biomass, lignocellulolytic enzyme production and lignocellulose degradation patterns by Auricularia auricula during solid state fermentation of corn stalk residues under different pretreatments. Food Chemistry, 384, 132622. https://doi.org/10.1016/j.foodchem.2022.132622 [17] Marina Tišma, Anita Juri?, Ana Buci?-Koji?, Mario Panji?ko and Mirela Planini?(2018) Biovalorization of brewers’ spent grain for the production of laccase and polyphenols J. Inst. Brew. 124: 182–186 [18] Mishra, A., Kumar, S., & Kumar Pandey, A. (2011). Laccase production and simultaneous decolorization of synthetic dyes in unique inexpensive medium by new isolates of white rot fungus. International Biodeterioration & Biodegradation, 65(3), 487–493. https://doi.org/10.1016/j.ibiod.2011.01.011 [19] Moran-Aguilar, M. G., Costa-Trigo, I., Calderón-Santoyo, M., Domínguez, J. M., & Aguilar-Uscanga, M. G. (2021). Production of cellulases and xylanases in solid-state fermentation by different strains of Aspergillus niger using sugarcane bagasse and brewery spent grain. Biochemical Engineering Journal, 172, 108060. https://doi.org/10.1016/j.bej.2021.108060 [20] Namnuch, N., Thammasittirong, A., & Thammasittirong, S. N.-R. (2021). Lignocellulose hydrolytic enzymes production by Aspergillus flavus KUB2 using submerged fermentation of sugarcane bagasse waste. Mycology, 12(2), 119–127. https://doi.org/10.1080/21501203.2020.1806938 [21] Nunes , C. S., and Kunamneni, A. (2018). “Chapter 7– laccases—properties and applications,” in Enzymes in Human and Animal Nutrition, eds C. S. Nunes, and V.Kumar(Cambridge,MA:AcademicPress),133–161. doi: 10.1016/b978 0-12-805419-2.00007-1 [22] Perdani, M. S., Margaretha, G., Sahlan, M., & Hermansyah, H. (2020). Solid state fermentation method for production of laccase enzyme with bagasse, cornstalk and rice husk as substrates for adrenaline biosensor. Energy Reports, 6, 336–340. https://doi.org/10.1016/j.egyr.2019.08.065 [23] Rajchenberg, M., De Errasti, A., & Gorjón, S. P. (2024). The genus Peniophora (Russulales, Basidiomycota) from Patagonia revisited. Mycological Progress, 23(1), 50. https://doi.org/10.1007/s11557-024-01989-7 [24] Rastogi, S., Soni, R., Kaur, J., & Soni, S. K. (2016). Unravelling the capability of Pyrenophora phaeocomes S-1 for the production of ligno-hemicellulolytic enzyme cocktail and simultaneous bio-delignification of rice straw for enhanced enzymatic saccharification. Bioresource Technology, 222, 458–469. https://doi.org/10.1016/j.biortech.2016.10.012 [25] Sharma, D., Garlapati, V. K., & Goel, G. (2016). Bioprocessing of wheat bran for the production of lignocellulolytic enzyme cocktail by Cotylidia pannosa under submerged conditions. Bioengineered, 7(2), 88–97. https://doi.org/10.1080/21655979.2016.1160190 [26] Singh, J., Kumar, P., Saharan, V., & Kapoor, R. K. (2019). Simultaneous laccase production and transformation of bisphenol-A and triclosan using Trametes versicolor. 3 Biotech, 9(4), 129. https://doi.org/10.1007/s13205-019-1648-1 [27] Soccol, C. R., Costa, E. S. F. D., Letti, L. A. J., Karp, S. G., Woiciechowski, A. L., & Vandenberghe, L. P. D. S. (2017). Recent developments and innovations in solid state fermentation. Biotechnology Research and Innovation, 1(1), 52–71. https://doi.org/10.1016/j.biori.2017.01.002 [28] Umar, A. (2021). Screening and evaluation of laccase produced by different Trichoderma species along with their phylogenetic relationship. Archives of Microbiology, 203(7), 4319–4327. https://doi.org/10.1007/s00203-021-02420-5 [29] Xu, Y.-L., Tian, Y., & He, S.-H. (2023). Taxonomy and Phylogeny of Peniophora Sensu Lato (Russulales, Basidiomycota). Journal of Fungi, 9(1), 93. https://doi.org/10.3390/jof9010093 [30] Zeko-Piva?, A., Tišma, M., Žnidarši?-Plazl, P., Kulisic, B., Sakellaris, G., Hao, J., & Planini?, M. (2022). The Potential of Brewer’s Spent Grain in the Circular Bioeconomy: State of the Art and Future Perspectives. Frontiers in Bioengineering and Biotechnology, 10, 870744. https://doi.org/10.3389/fbioe.2022.870744
Copyright
Copyright © 2025 Komal Solanke, Aparna Taware . This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET66953
Publish Date : 2025-02-14
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

